
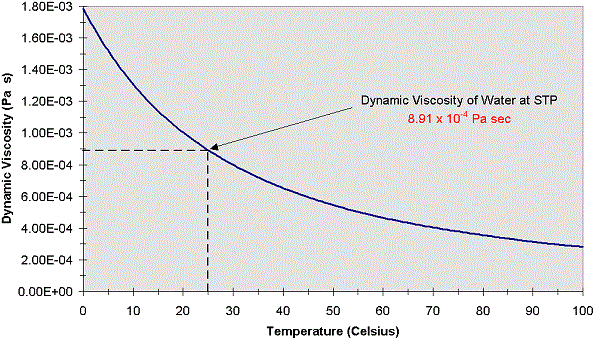
Understanding the distinction comes into play in industrial settings as record data is normally reported as kinematic viscosity, but onsite measurements are often measured as dynamic viscosity. Kinematic viscosity is not the same as dynamic viscosity, although they are related mathematically by the density of the fluid. This intermolecular friction is what makes the fluid resistant to change in shape and explains the thick and slow moving properties of highly viscous fluids. The stronger the intermolecular force, the more the molecules will stick together as they collide, manifesting a frictional interaction. Strongly polarized interactions like hydrogen bonding in water or interactions between large non-polar surfaces in viscous oils produce stronger intermolecular forces. Viscosity results from the intermolecular forces between molecules in a liquid. So, viscosity of liquids decreases when the temperature increases.Corrosionpedia Explains Kinematic Viscosity
In case of liquids, when the temperature increases the distance between molecules increases and the cohesive force decreases. Variation of Viscosity Unit with respect o temperature for some common fluids are given in fig 1.2(b). The fluids which follow this law is known as Newtonian, fluids otherwise, it is known as Non-Newtonian fluids. The constant of proportionality is called coefficient of viscosity.

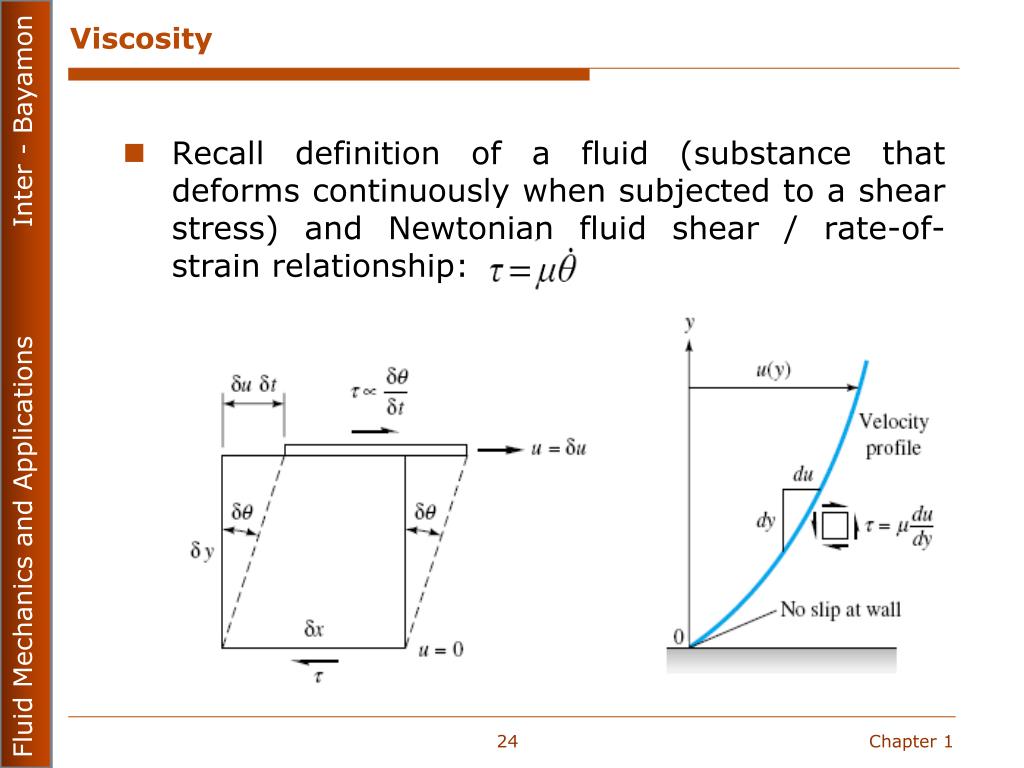
It states that the shear stress τ on fluid element, layer is directly proportional to the rate of shear strain. Since, water has a viscosity of 1, it is taken as standard substance for relative viscosity. Relative or Specific viscosity is the ratio of dynamic viscosity of any fluid to the dynamic viscosity of water at 20☌. In case of gases, it increases with increase in temperature. In case of liquids, kinematic viscosity decreases with increase in temperature. Likewise kinematic viscosity also involves the magnitudes of length and rime only. The name kinematic viscosity has been given to the ratio (µ/p) because kinematics is defined as the study of motion without regard to the cause of motion and it concerned with length and time only. The kinematic viscosity ( v) viscosity is defined as the ratio of dynamic viscosity to mass density. Dynamic Viscosity (μ):Īs explained earlier, the dynamic viscosity (μ) is defined as the shear stress required causing unit rate of shear deformation. Its unit can be derived asĭu/dy – is the rate of shear deformation or rate of shear strain. Μ (Mu) is the constant of proportionality or co-efficient of dynamic viscosity or Viscosity Unit. A fluid layer at a distance of y from surface moves with a velocity of `u’ and a layer at a distance of dy from y moves with a velocity of u+du.Īccording to Newton’s law of viscosity the shear force, F acting between two layers of fluid is proportional to difference in their velocities du and area A of the plate and inversely proportional to the distance dy between them. The plate moves with a velocity U by a force F as shown in fig 1.2. The space in between is filled with a fluid. Viscosity increases with increase in temperature in case of gases whereas it decreases in case of liquid.Ĭonsider, a plate is placed at a distance of ‘Y’ from the fixed surface. Viscosity can also be defined as the property of a fluid due to which it offers resistance to the movement of one layer of fluid over another adjacent layer.


 0 kommentar(er)
0 kommentar(er)
